Introduction
Lead-acid batteries are one of the most common types of batteries used in various applications. Understanding the basic principle of lead-acid batteries is necessary to make good use of them in various applications, such as automotive or uninterruptible power sources. Elevating familiarity with these concepts can enhance one’s ability to maintain them properly while maximizing their potential for optimal performance on demand across different settings/contexts. This article explores the chemical reactions in the ion flow mechanism necessary for controlling the charging and discharging processes.
Chemical Reaction of the Positive and Negative Electrodes
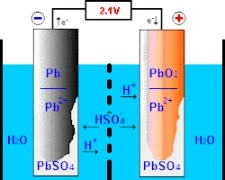
The lead-acid batterys’ operation entails chemical reactions at its two primary electrodes – the positive electrode, composed of lead dioxide, and the negative electrode, pure lead. Understanding the battery’s functioning begins with its design and construction.
When exposed to sulfuric acid along with another compound called “lead dioxide” at the positive electrode, this procedure results in the production of “oxygen ions” as well as an amalgamation called “lead sulfate.” At an equal pace, Pure lead combines with sulphuric acid to form hydrogen ions and yields lead sulfate. As a result of the previously described series of events, chemical energy is converted into electrical energy, which is used for various purposes in modern society.
The Flow of Ions in an Electrolyte Solution
It is fundamental to introduce an electrolyte solution to enable effective ion exchange between positively and negatively marked electrodes. This solution frequently comprises water mixed with sulfuric acid acting as the carrier for these ions. This critical compound can support proper battery function through its sulfate ions (SO₄²⁻), which actively migrate throughout each charge and discharge.
While in charging mode, electric current facilitates movement movements of charged sulfur compounds that are dissolved in electrolytes within a typical battery across positively charged traces, transitioning towards negatively charged regions or traces using electrolytic action. This transformational activity reverses conversion formation that hindered previous storage by transforming solid-to-fluid states through the Charge-Discharge cycle. Gradual restoring, therefore, enables these batteries to become capable enough for securely storing electrical charges with diverse storage capabilities across various applications.
Schematic and Description of the Charge and Discharge Process
Understanding precisely how rechargeable batteries, like those using the lead-acid design recharge, might seem complicated but can be more manageable when visualized using schematics descriptions. In essence, we observe that charging requires powering up from an external electrical supply so that an internal chemical reaction can take place wherein inactive regions like Lead Sulfate rearrange themselves into active chemicals again, like Pure Lead or Lead Dioxide – allowing for renewed energy storage in those cells of our rechargeable batteries.
As it discharges electric energy into an external circuit, a battery undergoes a chemical transformation: its sulfuric acid reacts with pure lead and lead dioxide to make copious amounts of lead sulfate. This process steadily consumes the stored power of the device till its voltage diminishes completely, requiring immediate recharging for further usage regularly.
The Bottom Line
In brief, knowledge about how lead acid batteries work is fundamental to maximizing their productivity and longevity. The key factor contributing to its overall functionality is the multitude of chemical reactions occurring at both poles in conjunction with ion flow throughout its electrolytic solution during charge-discharge cycles.